Why Does Steam Distillation Lower Boiling Point . Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to.
from www.mdpi.com
Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point.
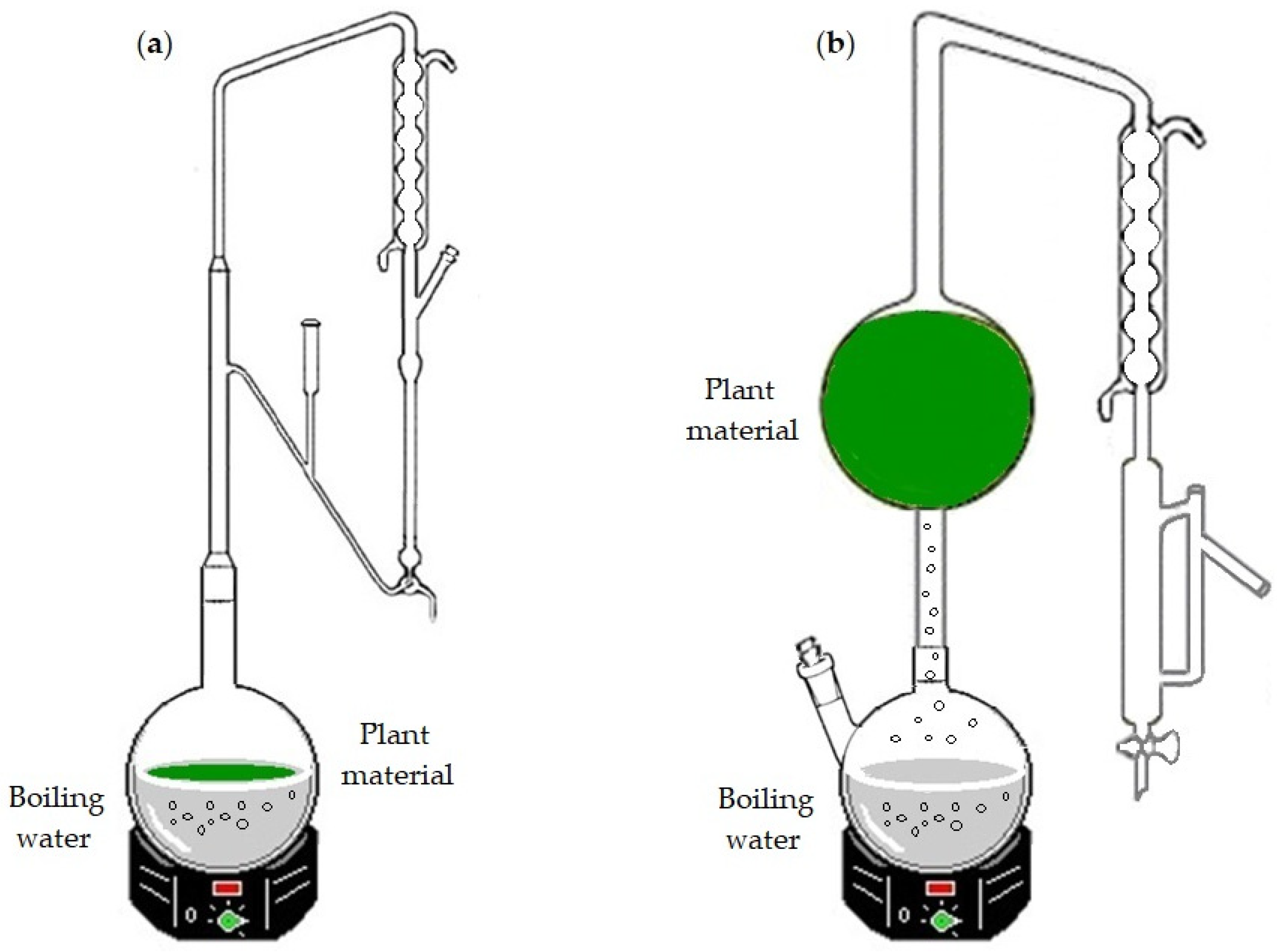
Separations Free FullText Essential Oil Variability of Azorean
Why Does Steam Distillation Lower Boiling Point Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids.
From mungfali.com
Phase Diagram Boiling Point Why Does Steam Distillation Lower Boiling Point Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point.. Why Does Steam Distillation Lower Boiling Point.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web this behavior. Why Does Steam Distillation Lower Boiling Point.
From www.coursehero.com
[Solved] Organic Chemistry Laboratory Activity Data/Procedure for Why Does Steam Distillation Lower Boiling Point Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor. Why Does Steam Distillation Lower Boiling Point.
From www.britannica.com
distillation summary Britannica Why Does Steam Distillation Lower Boiling Point Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the. Why Does Steam Distillation Lower Boiling Point.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web agitated mixtures. Why Does Steam Distillation Lower Boiling Point.
From www.mdpi.com
Separations Free FullText Essential Oil Variability of Azorean Why Does Steam Distillation Lower Boiling Point Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point.. Why Does Steam Distillation Lower Boiling Point.
From brainly.in
Explain the process of simple distillation with diagram Brainly.in Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature.. Why Does Steam Distillation Lower Boiling Point.
From mavink.com
Elevation In Boiling Point Graph Why Does Steam Distillation Lower Boiling Point Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),.. Why Does Steam Distillation Lower Boiling Point.
From www.numerade.com
SOLVED (6). Define distillation? boiling liquids condensing liquids Why Does Steam Distillation Lower Boiling Point Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point. Why Does Steam Distillation Lower Boiling Point.
From neutrium.net
Distillation Fundamentals Neutrium Why Does Steam Distillation Lower Boiling Point Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the. Why Does Steam Distillation Lower Boiling Point.
From www.chemengghelp.com
Steam Distillation Method for Component Separation ChemEnggHelp Why Does Steam Distillation Lower Boiling Point Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids.. Why Does Steam Distillation Lower Boiling Point.
From www.yaclass.in
Fractional Distillation — lesson. Science CBSE, Class 9. Why Does Steam Distillation Lower Boiling Point Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web agitated mixtures of immiscible. Why Does Steam Distillation Lower Boiling Point.
From www.umsl.edu
Distillation Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature.. Why Does Steam Distillation Lower Boiling Point.
From slidetodoc.com
Properties of matter Pure Substances Mixtures Solutions Separation Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web steam distillation, or steam. Why Does Steam Distillation Lower Boiling Point.
From triptonkosti.ru
Диаграмма метанол вода 81 фото Why Does Steam Distillation Lower Boiling Point Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower temperature. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point.. Why Does Steam Distillation Lower Boiling Point.
From www.askiitians.com
Organic Chemistry Purification of organic compounds askIITians Why Does Steam Distillation Lower Boiling Point Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web agitated mixtures of immiscible liquids will boil at a temperature lower than the boiling point of either of the pure liquids. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can. Why Does Steam Distillation Lower Boiling Point.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Why Does Steam Distillation Lower Boiling Point Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web the distilling temperature in steam distillation is always below \(100^\text{o} \text{c}\) (the boiling point of water),. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower. Why Does Steam Distillation Lower Boiling Point.
From solutionpharmacy.in
Steam Distillation How it Works? Why Does Steam Distillation Lower Boiling Point Web steam distillation is used to distil compounds at a temperature lower than the normal boiling point. Web steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to. Web this behavior occurs because a lower vapor pressure is necessary for boiling, which can be achieved at a lower. Why Does Steam Distillation Lower Boiling Point.